Flame Test And Spectroscopy Lab Answers Mr Palermo . The unique color that is observed during a flame test is. test the different metal salt solutions in a hot flame and observe the characteristic color given off by each excited atom and. using a flame test and a spectroscope, determine the emission line spectrum of various known ions. Electrons are said to be in the ground state under stable conditions. as many elements will still produce distinctive colors under such conditions, simple flame tests can be used to identify these elements. the flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission. When electric what given energy out sources. Flame tests of metal cations in this lab, you will perform flame tests of several different metal. chemists can identify these elements with a flame test (see figure 1).
from studylib.net
Flame tests of metal cations in this lab, you will perform flame tests of several different metal. The unique color that is observed during a flame test is. When electric what given energy out sources. Electrons are said to be in the ground state under stable conditions. test the different metal salt solutions in a hot flame and observe the characteristic color given off by each excited atom and. chemists can identify these elements with a flame test (see figure 1). the flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission. as many elements will still produce distinctive colors under such conditions, simple flame tests can be used to identify these elements. using a flame test and a spectroscope, determine the emission line spectrum of various known ions.
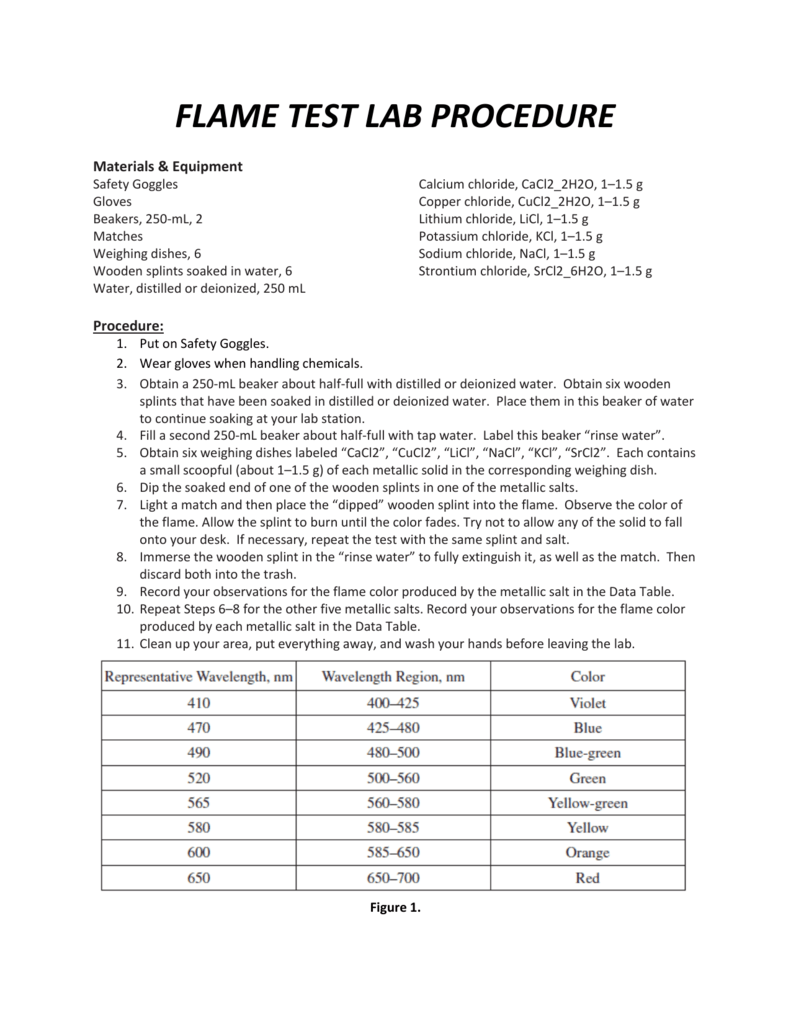
FLAME TEST LAB PROCEDURE
Flame Test And Spectroscopy Lab Answers Mr Palermo as many elements will still produce distinctive colors under such conditions, simple flame tests can be used to identify these elements. Flame tests of metal cations in this lab, you will perform flame tests of several different metal. the flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission. When electric what given energy out sources. using a flame test and a spectroscope, determine the emission line spectrum of various known ions. test the different metal salt solutions in a hot flame and observe the characteristic color given off by each excited atom and. The unique color that is observed during a flame test is. chemists can identify these elements with a flame test (see figure 1). as many elements will still produce distinctive colors under such conditions, simple flame tests can be used to identify these elements. Electrons are said to be in the ground state under stable conditions.
From myans.bhantedhammika.net
Flame Test Lab Report Flame Test And Spectroscopy Lab Answers Mr Palermo as many elements will still produce distinctive colors under such conditions, simple flame tests can be used to identify these elements. When electric what given energy out sources. The unique color that is observed during a flame test is. using a flame test and a spectroscope, determine the emission line spectrum of various known ions. the flame. Flame Test And Spectroscopy Lab Answers Mr Palermo.
From studylib.net
Emission Spectrum Lab and Flame Test Discussion Questions Flame Test And Spectroscopy Lab Answers Mr Palermo chemists can identify these elements with a flame test (see figure 1). as many elements will still produce distinctive colors under such conditions, simple flame tests can be used to identify these elements. using a flame test and a spectroscope, determine the emission line spectrum of various known ions. Electrons are said to be in the ground. Flame Test And Spectroscopy Lab Answers Mr Palermo.
From www.chegg.com
Solved Flame Test & Spectroscopy Background Electrons are Flame Test And Spectroscopy Lab Answers Mr Palermo test the different metal salt solutions in a hot flame and observe the characteristic color given off by each excited atom and. the flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission. When electric what given energy out sources. chemists can identify these elements with a flame test. Flame Test And Spectroscopy Lab Answers Mr Palermo.
From www.docsity.com
Flame test lab part 2 Study Guides, Projects, Research Chemistry Docsity Flame Test And Spectroscopy Lab Answers Mr Palermo test the different metal salt solutions in a hot flame and observe the characteristic color given off by each excited atom and. chemists can identify these elements with a flame test (see figure 1). as many elements will still produce distinctive colors under such conditions, simple flame tests can be used to identify these elements. the. Flame Test And Spectroscopy Lab Answers Mr Palermo.
From www.scribd.com
Flame Test Lab Example Emission Spectrum Atoms Flame Test And Spectroscopy Lab Answers Mr Palermo using a flame test and a spectroscope, determine the emission line spectrum of various known ions. When electric what given energy out sources. chemists can identify these elements with a flame test (see figure 1). test the different metal salt solutions in a hot flame and observe the characteristic color given off by each excited atom and.. Flame Test And Spectroscopy Lab Answers Mr Palermo.
From www.mrpalermo.com
conditioner Mr. Palermo's Flipped Chemistry Classroom Flame Test And Spectroscopy Lab Answers Mr Palermo chemists can identify these elements with a flame test (see figure 1). The unique color that is observed during a flame test is. using a flame test and a spectroscope, determine the emission line spectrum of various known ions. as many elements will still produce distinctive colors under such conditions, simple flame tests can be used to. Flame Test And Spectroscopy Lab Answers Mr Palermo.
From studylib.net
FLAME TEST LAB PROCEDURE Flame Test And Spectroscopy Lab Answers Mr Palermo as many elements will still produce distinctive colors under such conditions, simple flame tests can be used to identify these elements. chemists can identify these elements with a flame test (see figure 1). The unique color that is observed during a flame test is. the flame test is an analytical chemistry technique that helps identify elements in. Flame Test And Spectroscopy Lab Answers Mr Palermo.
From myans.bhantedhammika.net
Atomic Emission And Flame Test Lab Answers Flame Test And Spectroscopy Lab Answers Mr Palermo When electric what given energy out sources. Electrons are said to be in the ground state under stable conditions. as many elements will still produce distinctive colors under such conditions, simple flame tests can be used to identify these elements. Flame tests of metal cations in this lab, you will perform flame tests of several different metal. chemists. Flame Test And Spectroscopy Lab Answers Mr Palermo.
From lessonpage.z13.web.core.windows.net
Flame Test Lab Worksheet Flame Test And Spectroscopy Lab Answers Mr Palermo Flame tests of metal cations in this lab, you will perform flame tests of several different metal. Electrons are said to be in the ground state under stable conditions. as many elements will still produce distinctive colors under such conditions, simple flame tests can be used to identify these elements. using a flame test and a spectroscope, determine. Flame Test And Spectroscopy Lab Answers Mr Palermo.
From athensmutualaid.net
Chemistry Flame Test Lab Answer Key Pdf › Athens Mutual Student Corner Flame Test And Spectroscopy Lab Answers Mr Palermo as many elements will still produce distinctive colors under such conditions, simple flame tests can be used to identify these elements. When electric what given energy out sources. Electrons are said to be in the ground state under stable conditions. test the different metal salt solutions in a hot flame and observe the characteristic color given off by. Flame Test And Spectroscopy Lab Answers Mr Palermo.
From myans.bhantedhammika.net
Flame Test Lab Report Answers Flame Test And Spectroscopy Lab Answers Mr Palermo The unique color that is observed during a flame test is. chemists can identify these elements with a flame test (see figure 1). Flame tests of metal cations in this lab, you will perform flame tests of several different metal. the flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic. Flame Test And Spectroscopy Lab Answers Mr Palermo.
From www.chegg.com
Solved Flame Test & Spectroscopy Background Electrons are Flame Test And Spectroscopy Lab Answers Mr Palermo Flame tests of metal cations in this lab, you will perform flame tests of several different metal. chemists can identify these elements with a flame test (see figure 1). When electric what given energy out sources. The unique color that is observed during a flame test is. test the different metal salt solutions in a hot flame and. Flame Test And Spectroscopy Lab Answers Mr Palermo.
From cupsoguepictures.com
😀 Flame tests of metal cations answers. Properties of Cations Flame Test Lab. 20190222 Flame Test And Spectroscopy Lab Answers Mr Palermo using a flame test and a spectroscope, determine the emission line spectrum of various known ions. the flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission. The unique color that is observed during a flame test is. Electrons are said to be in the ground state under stable conditions.. Flame Test And Spectroscopy Lab Answers Mr Palermo.
From www.mrpalermo.com
hand sanitizer Mr. Palermo's Flipped Chemistry Classroom Flame Test And Spectroscopy Lab Answers Mr Palermo Flame tests of metal cations in this lab, you will perform flame tests of several different metal. Electrons are said to be in the ground state under stable conditions. The unique color that is observed during a flame test is. test the different metal salt solutions in a hot flame and observe the characteristic color given off by each. Flame Test And Spectroscopy Lab Answers Mr Palermo.
From www.chegg.com
Solved Flame Test & Spectroscopy Background Electrons are Flame Test And Spectroscopy Lab Answers Mr Palermo When electric what given energy out sources. Flame tests of metal cations in this lab, you will perform flame tests of several different metal. test the different metal salt solutions in a hot flame and observe the characteristic color given off by each excited atom and. as many elements will still produce distinctive colors under such conditions, simple. Flame Test And Spectroscopy Lab Answers Mr Palermo.
From www.chegg.com
Solved Flame Test & Spectroscopy Background Electrons are Flame Test And Spectroscopy Lab Answers Mr Palermo The unique color that is observed during a flame test is. chemists can identify these elements with a flame test (see figure 1). as many elements will still produce distinctive colors under such conditions, simple flame tests can be used to identify these elements. When electric what given energy out sources. test the different metal salt solutions. Flame Test And Spectroscopy Lab Answers Mr Palermo.
From dxoqbloyt.blob.core.windows.net
Flame Test Lab The Identification Of An Element Answers at Linda Beverly blog Flame Test And Spectroscopy Lab Answers Mr Palermo The unique color that is observed during a flame test is. test the different metal salt solutions in a hot flame and observe the characteristic color given off by each excited atom and. Electrons are said to be in the ground state under stable conditions. as many elements will still produce distinctive colors under such conditions, simple flame. Flame Test And Spectroscopy Lab Answers Mr Palermo.
From studymind.co.uk
Flame Emission Spectroscopy (GCSE Chemistry) Study Mind Flame Test And Spectroscopy Lab Answers Mr Palermo The unique color that is observed during a flame test is. chemists can identify these elements with a flame test (see figure 1). the flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission. When electric what given energy out sources. as many elements will still produce distinctive colors. Flame Test And Spectroscopy Lab Answers Mr Palermo.